Background
The HLA class I ELISA is an enzyme immunoassay based on the detection of β2-microglobulin subunit of HLA class I complexes, after capturing the complex through the conjugated biotin. To this end, biotinylated HLA class I complex is first captured in streptavidin coated microtiter wells. Subsequently, HRP-conjugated anti-human β2- microglobulin is added to detect intact HLA class I complexes. Only intact HLA class I complexes are recognized. Peptides with high affinity binding will be clearly detected by this ELISA technique, while peptides with a moderate to low binding affinity for HLA class I provide a moderate to non-detectable signal. This protocol is designed to evaluate the efficiency of peptide exchange when using the Flex-T™ system.
Reagent List:
- Coating Buffer 5X concentrate (Cat# 421701)
- Streptavidin solution at 1 mg/ml (BioLegend Cat# 280302)
- Wash Buffer 20X concentrate (BioLegend Cat# 421601): After dilution with DI water the buffer is 1X PBS containing 0.05% (v/v) Tween 20
- Dilution Buffer 10X concentrate (1 M NaCl, 0.5M Tris, 1% BSA, 0.2% Tween 20, pH8.0. See Appendix 1)
- HRP anti-human β2-microglobulin antibody (Cat# 280303)
- Flex-T™ ELISA Positive Control (BioLegend Cat# 280301)
- Plate sealers (BioLegend Cat# 423601)
- Deionized (DI) water
|
- Substrate Buffer, 10X concentrate (0.1 M citric acid monohydrate/tri-Sodium citrate dehydrate, pH 4.0, See Appendix 1)
- ABTS substrate solution (50X 40mM solution made from e.g. Sigma Cat#A1888, or equivalent. See Appendix 1)
- Hydrogen peroxide solution (e.g. Sigma Cat#H3410, or equivalent. See Appendix 1)
- Stop Solution (2% [w/v] oxalic acid dehydrate, e.g. Sigma Cat#247537. See Appendix 1)
- Nunc™ MaxiSorp™ ELISA Plates, Uncoated (BioLegend Cat# 423501)
|
Equipment
- Incubator (37°C)
- ELISA plate shaker
- Wash bottle or automated microplate washer
|
- Microtiter plate reader for measuring absorbance at 414nm
- Adjustable pipettes to measure volumes ranging from 3µl to 1ml
- Multichannel pipetting devices
|
Reagent Preparation
See Appendix 1 for recipes and recommended reagents. All reagents should be prepared or diluted immediately prior to use. Before use, bring all reagents to room temperature (18-25°C) with the exception of the HRP anti-human β2-microglobulin antibody and the ELISA positive control which have to be kept on melting ice to ensure stability. Sodium azide inactivates HRP, do not use sodium azide-containing solutions, nor add sodium azide to the supplied materials. Centrifuge all vials before use (1 minute 3000xg at 4°C). Do not allow wells to stand uncovered or dry for extended periods between incubation steps.
Note: Protocol updated July 31, 2019. Previous version recommended a 1200-fold dilution of the exchange reaction mixture in step 13. Current version recommends a 1400-fold dilution.
Protocol
- Dilute the 5X Coating Buffer to 1X with DI water
- Prepare Streptavidin solution to coat the plate: 24µl of Streptavidin solution 11.976 ml 1X Coating Buffer
- Calculate the amount of 1X Dilution Buffer required and prepare the solution by diluting the 10X concentrated buffer 10 times in DI water before use. The 1X Dilution Buffer can be stored for up to one week at 2-8°C.
- Prepare Wash Buffer; dilute the 20X wash buffer with DI water, at least 300ml per plate.
- Dilute concentrated HRP-conjugated antibody to 0.3µg/ml in 1X Dilution Buffer just before use. Prepare 12ml per plate.
- Prepare the substrate solution approximately 10 minutes before use:
- 10.34ml DI water
- 1.2ml of Substrate Buffer 10X concentrate
- 240µl of ABTS stock solution
- 120µl of Hydrogen peroxide solution
- Vortex to mix well
- The substrate solution should be at room temperature (18-25°C) for optimal reproducible results.
- Dilute the Flex-T™ ELISA Positive Control to prepare a 2.7µM solution:
- 5µl of Flex-T™ ELISA Positive Control at 0.2mg/ml
- 2.9µl of 1X Dilution Buffer
Assay Procedure
- Coat the wells of the plate with Streptavidin. Add 100µl of Streptavidin solution to all wells. Seal the plate and incubate overnight (16-18 hrs) at room temperature (18-25°C).
- Discard the coating solution and wash the plate 3 times with at least 300µl Wash Buffer per well and blot residual buffer by firmly tapping plate upside down on absorbent paper. All subsequent washes should be performed similarly.
- To block non-specific binding and reduce background, add 300µl of 1X Dilution Buffer to all wells.
- Seal the plate and incubate for 30 minutes at room temperature (18-25°C).
- Prepare three dilutions of the HLA control by serial dilution in 1X Dilution Buffer. Prepare the controls fresh and keep them on melting ice until usage.
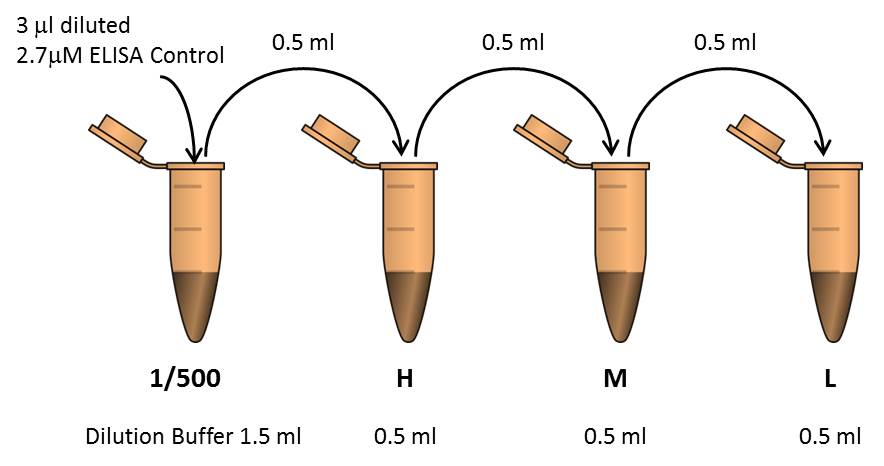
- To evaluate the outcome of UV-mediated HLA peptide exchange, dilute a small aliquot of the exchange reaction mixture 1400-fold in 1X Dilution Buffer (refer to the peptide exchange step in Protocol for fluorescent Flex-T™ generation and antigen specific CD8+ T cell staining). Mix thoroughly.
- Discard the Dilution Buffer from the plate and blot the residual buffer.
- Pipette 100µl of 1X Dilution Buffer (blank), diluted ELISA controls (H, M, L, positive, negative, and UV only), or exchange reaction mixture dilutions, into the appropriate wells (recommended in duplicate, see plate map).
- Seal the plates and incubate for 1 hour at 37°C.
- Discard the liquid from the wells and wash 3 times with 300µl of wash buffer per well.
- Add 100µl of diluted HRP-conjugated antibody.
- Seal the plates and incubate for 1 hour at 37°C.
- Discard the liquid from the wells and wash 3 times with 300µl of wash buffer per well.
- Add 100µl of substrate solution.
- Incubate for 8 minutes at room temperature (18-25°C) in the dark on a plate shaker at 400-500 rpm
- Add 50µl of Stop Solution to all wells and read at 414nm in an ELISA reader within 30 minutes.
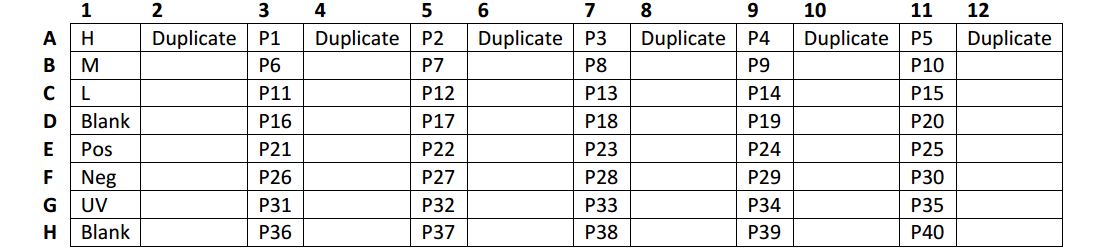
Plate map example for the HLA class I ELISA:
Pos: Positive exchange control peptide (See table in appendix 1)
Neg: Negative exchange control peptide (See table in appendix 1)
UV: UV-illuminated conditional HLA complex in the absence of exchange peptide
Blank: 1X Dilution Buffer
H: HLA control H
M: HLA control M
L: HLA control L
P: peptide of choice
Representative Results:
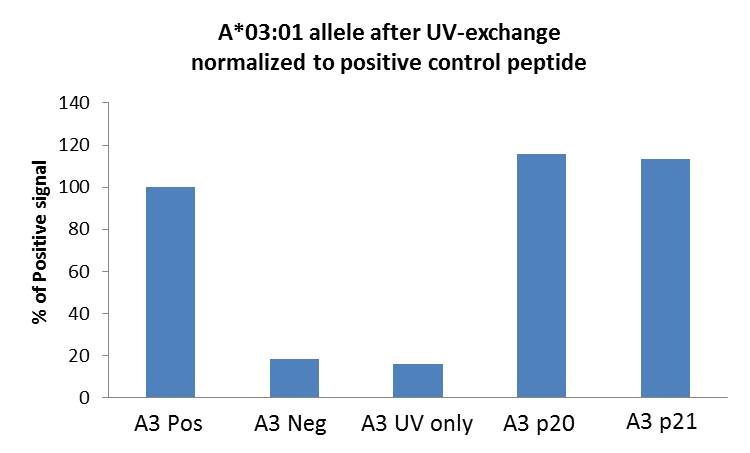 |
The bar graph shows peptide exchange of HLA-A*03:01 allele. Monomers were irradiated with UV light in the presence of positive (Pos), negative (Neg), p20 (EBV RLRAEAQVK), and p21 (EBV RVRAYTYSK) peptides, or no peptide (UV only). ELISA signal was normalized using the Pos control well. H, M, and L controls not shown. |
Appendix 1.
Note: Scale up or down as needed, indicated measurements are only guidelines.
50X ABTS stock solution: 40mM, ABTS (2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (Sigma Cat# A1888)
Dissolve 2.195g solid in 100ml filtered DI water. Store at 2-8°C.
100X Hydrogen peroxide stock solution: 0.6% H2O2 Dilute 1ml 30% H2O2 (Sigma Cat# H3410) in 49ml filtered DI water. Store at 2-8°C.
10X Dilution Buffer:1 M NaCl, 0.5 M Tris, 1% BSA, 0.2% Tween 20, pH 8.0
- Dissolve 29.22g NaCl (Sigma Cat# S7653) and 30.29g Tris base (Sigma Cat# T1503) in 400ml DI water; adjust pH to 8.0 with HCl
- Dissolve 5g BSA (Sigma; cat# A7030) in this solution
- Add 1ml Tween-20 (Sigma Cat# p7949) mix gently
- Adjust final volume to 500ml
- Filter and store at 2-8°C
10X Substrate Buffer:0.1 M citric acid monohydrate / tri-Sodium citrate dihydrate, pH 4.0
- Make 0.1M Citric Acid: dissolve 3.84g Citric Acid (Sigma Cat#C2404) in 200ml DI water. Store at 2-8°C
- Make 0.1M Tri-Sodium Citrate dihydrate: dissolve 5.88g Tri-sodium citrate dihydrate (Sigma Cat# S1804) in 200 DI water. Store at 2-8°C
- Mix 59ml 0.1M Citric Acid solution with 41ml 0.1M Tri-sodium citrate dihydrate solution. Filter, store at 2-8°C.
Stop buffer ready for use: 2% (w/v) oxalic acid dihydrate Dissolve 2g oxalic acid dihydrate (Sigma cat #247537) in 100ml DI H2O. Store at RT
Sequence of positive and negative peptide controls:



